Researchers from the CNAG participate in a study, led by IDIBAPS and the University of Barcelona and published today in Nature Genetics, which reveals an unexpected connection between epigenetic changes associated with lymphocytes maturation and those observed in cancer. Both processes share similar changes in large regions of the genome.
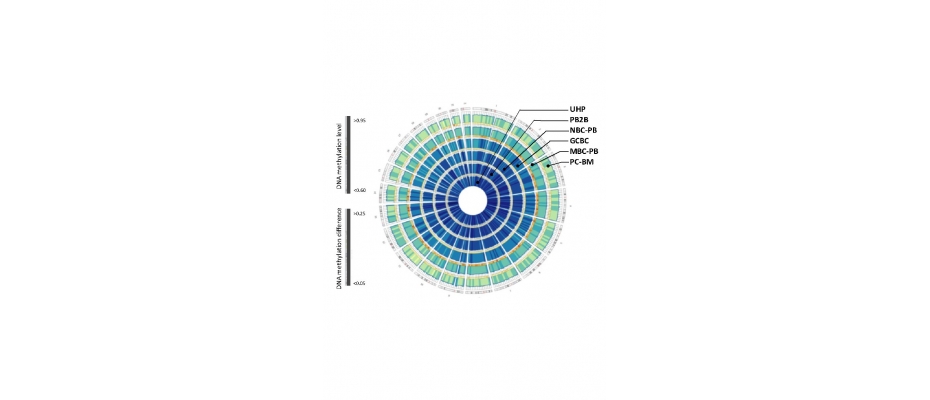
The work, led by Iñaki Martín-Subero, is the first to analyze the epigenome during cell maturation of B-lymphocytes, the immune system cells responsible for antibody production, and provides epigenetic maps of each step in this process.
To decipher how the epigenome changes as the cells mature, the participation of the CNAG has been essential. According to Ivo Gut, Director of CNAG, "this study has required the application of advanced mass sequencing techniques and the development of new analysis methods”.
The Blueprint Project in the CNAG
The study is part of the Blueprint project, a large-scale research project funded by the EU with the participation of 42 researchers from 7 countries, with the principal aim to study the epigenome in detail in healthy and diseased human cells. Blueprint focuses on distinct types of haematopoietic cells from healthy individuals and on their malignant leukaemic counterparts. It aims to generate at least 100 reference epigenomes and study them to advance and exploit knowledge of the underlying biological processes and mechanisms in health and disease. This aim feeds into the overall objective of the International Human Epigenome Consortium (IHEC).
The CNAG has leading roles in the standardization and quality control of the sequencing assays used in the project, is the sole centre involved in the production and primary data analysis of the DNA methylation data from whole genome bisulphite sequencing, and is part of the multi-centre group working on the higher-level integrative analyses of the complete set of epigenomic data.
The CNAG also played a leading role in the analysis as well as in the data production of the first published Blueprint paper, in collaboration with the group of Iñaki Martín-Subero, investigating changes in genome wide DNA methylation patterns in a collection of Chronic Lymphocytic Leukaemia (CLL) samples (Kulis et. al., Nature Genetics 2012).
The dynamism of the Human epigenome
In 2001, after completing the sequence of the human genome, scientists realized that knowing the complete DNA sequence did not allow them to understand its role and how the same gene sequence could give rise to the multiple cell types that make up the body. From there arises the importance of studying the epigenome, which is anything that alters the expression of genes without changing the DNA strand.
The article published in Nature Genetics goes deeper into the epigenetic processes that occur during B-lymphocytes maturation and demonstrates that the human epigenome is much more dynamic than was previously thought: in the normal process of maturation of these cells the epigenome changes at 30% of possible sites affecting several million regions of the genome. The study also shows that in contrast to what has been published to date regarding DNA methylation, the main epigenetic mechanism, only a small proportion of changes in the degree of methylation are related to gene expression.
New approach in cancer epigenetics
Furthermore, this new study has revealed that more than half of epigenetic changes believed to specific to tumor cells are also observed in long-life blood cells. This unexpected finding challenges the current models of cancer epigenetics. "We have found an epigenetic signature in long life lymphocytes that was previously only associated with cancer cells. This article proposes a new integrative model in which cellular longevity, whether it occurs in the context of cancer, aging or healthy cells, is associated with similar epigenetic features”, explains Martin-Subero.
The study breaks new ground in the study of the immune system cells, aging and cancer and offers the scientific community a new tool with implications for both basic and translational research. Dr. Elias Campo, co-author of the study, stresses that "the greatest contribution of this work is that it provides a new vision that links the normal and cancer cell maturation and changes the way we perceive the epigenome of this disease”.
Work of reference:
Whole-genome fingerprint of the DNA methylome during human B cell differentiation.Nature Genetics.










